
The Intergovernmental Panel on Climate Change (IPCC) predicts the greenhouse gas concentration projected trajectory based on a set of scenarios known as the Representative Concentration Pathways (RCP). Sabine et al., (2004) reported that a third of the anthropogenic CO 2 (from activities such as fossil fuel burning and cement production) produced over the past 200 years has been absorbed by the ocean, resulting in a decrease in pH of 0.1 units. Ocean acidification is a chemical change caused primarily by the uptake of anthropogenic atmospheric CO 2 by the ocean, which results in an alteration in the chemistry of the seawater (Figure 1).
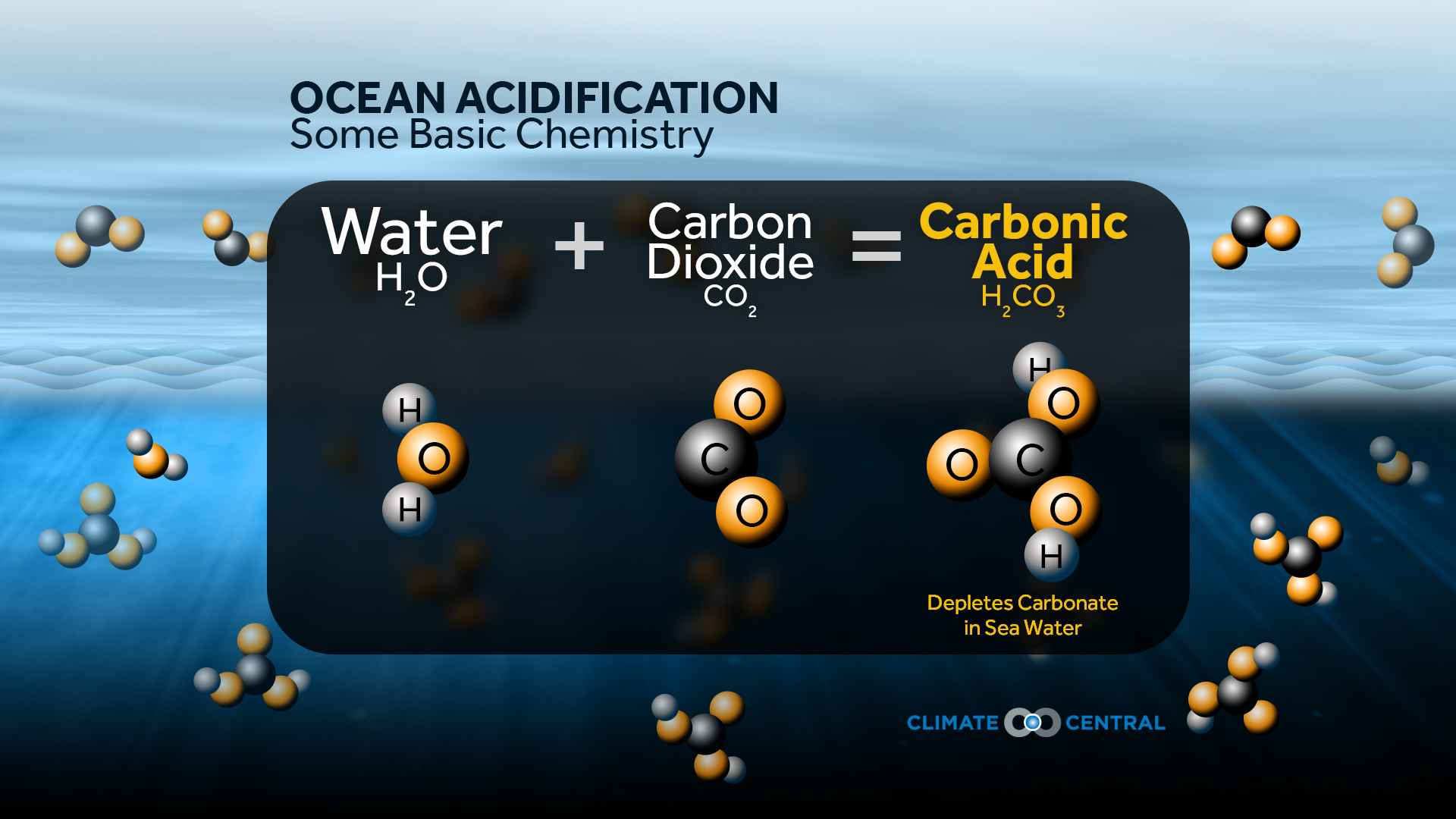
Calcifying organisms such as pelagic gastropods, bivalves and corals may be particularly affected (Figure 2).Ĭhanges to the ocean as a consequence of increasing atmospheric CO 2 and other greenhouse gases are impacting marine systems, resulting in both physical (such as increasing water temperatures) and chemical (such as decreasing pH) changes. The effects of the decrease in seawater pH and changes to the saturation states of calcium carbonates may be corrosive to the shells, skeletons and impact larval stages of some marine organisms. Although the input of carbon dioxide from the atmosphere has only small spatial variation, some marine regions will be more rapidly affected, with the susceptibility of seawater to change dependent on the chemical composition, depth and temperature (Hoegh-Guldberg et al., 2018). The pH is a logarithmic scale and this equates to an increase in acidity of approximately 30%. Sabine et al., (2004) reported that a third of the anthropogenic carbon dioxide produced since pre-industrial times has been absorbed by the ocean, resulting in a decrease in pH of approximately 0.1 units.

This is a ‘litmus paper’ diagram the colour changes from blue to red as more carbon dioxide (CO 2) is absorbed and the carbonate equilibria shift to release more hydrogen ions.Ĭalcium carbonate minerals such as calcite and aragonite are used by many marine organisms for production of their shells.


 0 kommentar(er)
0 kommentar(er)
